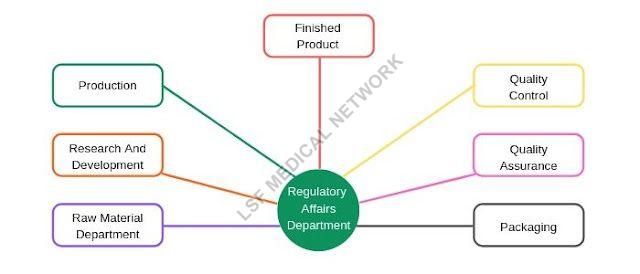
Regulatory Consultancy Services
Leading Manufacturers, Exporters, Wholesaler, Trader of Clinical and Non Clinical Research Service, Drug Regulatory Writing Services, Embassy Legalization Attestation For Pharmaceuticals Medical Devices, Pharma Analytical Support Services, Pharma Compliance for Medical Device, Pharma Regulatory Documentation and Pharmaceutical Translation Services from Noida.
We are one of the well-known names in the industry that is involved in offering trusted clinical & non clinical research services. We offer non-clinical research services through research laboratories. The biomedical or health-related research studies are performed on both human volunteers and animals. These researches are conducted to study certain diseases as well as to investigate about new methods to be used for the treatment of the known diseases. The research work is also done for analyzing the new potential treatments and medications. The service is conducted by our group or professionals belonging to the health care industry.
- We offer our service in the area of contract research for the formulation development, bio-assays, comparative analysis of ayurvedic formulations and FMCG products, standardization and quality control of herbal drugs, natural cosmetics, pro-biotic, ayurvedic and other traditional medicine.
- We provide collaborative clinical and/or non-clinical trial services fully complying the laws and prescribed standards.
- We understand that clinically validated formulations in FMCG & traditional medicines have more acceptability than its counterparts.
- We have a wide network of Clinical laboratories fully compliant with laws and standards. All our associated laboratories have DCGI approval and Good Clinical Practices (GCP) approval.
- Our clinical/non-clinical trial services are confluent with health care services aimed at providing customized solutions for complete healthcare, FMCG, wellness and pharmaceutical industry requirements.
- Our services are managed by a group of professionals from healthcare, research, and development, a decade of experience & expertise of these professionals ensure our clients the best possible consultancy and clinical services.
Drug regulatory writing services include a professional medical writing that includes providing high-quality documentation solutions across the pharmaceutical framework and commercialization spectrum. The drug regulatory writing has become a critical part of the pharmaceutical industry as the regulatory authorities have implemented a high structured review procedure which needs extensive documentation. The medical writing is essential as it plays an important role in the communication of scientific ideas. We assist in collecting, organizing, composing, editing, rearranging, and producing diverse medical and scientific documentations, which are needed to support the development of your product.
LSF MN is a growing name in the industry and offering services for embassy legalization attestation for pharmaceuticals medical devices. We are based in Noida (Uttar Pradesh, India) and can be trusted for handling documents, attestation, apostille & verification. A team of professionals is always there to assist you in your requirements and render prompt, reliable and cost-effective solutions. We tailor our services as per the individual’s needs. So, let us know your requirements and functional needs, we assure to work accordingly.
We assure :
- Efficient collection of documents & credentials,
- Timely completion of the procedures and
- Prompt deliveries at customer’s doorsteps, in shortest amount of time anywhere in the world.
We are the noteworthy names in the industry that is involved in offering pharma analytical support services. We are located in Noida (Uttar Pradesh, India). We offer a wide range of testing services to meet the research and development challenges of the industry. As we are supported by experienced professionals, we are capable of conducting any type of chemical and instrumental analysis. We assist our clients until the delivery of the complete analytical lab report with raw data. We also provide bioanalytical laboratory report, microbiology/biochemistry laboratory report, and many more.
LSF MN is a Noida (Uttar Pradesh, India) based service provider agency, offering complete assistance for pharma compliance for medical device requirements of the clients. We have a support of skilled individuals, who work in coordination with clients to meet and adhere to the norms of the industry. With our services, we perform several assessments to measure the operational parameters and provide consultancy to improve and maintain them. For free consultation, ring us on the given numbers.
Ours is a well-known name in the industry that is engaged in offering trusted pharma regulatory documentation services. We are capable of making the well-prepared regulatory application into smoother approval process as we have the resources to do so. Ours is thus considered as one-stop for all your regulatory requirements. We handle the documentation procure in an efficient manner without creating any hassle for the clients. Contact us to obtain our services anytime. We are located in Noida (Uttar Pradesh, India).
LSF MN is a noteworthy name if you are seeking professional aid for pharmaceutical translation requirements. Operating from Noida (Uttar Pradesh, India), we are providing pharmaceutical translation services across a large number of languages. With our services, we aim to simplify the procedure for our revered clients. So, let us know your area of operations and pharmaceutical translation requirements, we assure to work around them. For a detailed discussion, ring us on the given numbers.
We at LSF MEDICAL NETWORK ensure that :
- your project is allotted to the best professional for the job in our vast network of 5000+native translators so that your pharmaceuticals industry translation is reliable and accurate every time.
- every document is thoroughly proof-read with our proprietary real-time review system.
- correctness, clarity and consistency are deliberately achieved.
At LSF, we deal with the following Pharmaceutical translations on daily basis :
- Adverse events reports
- Standard Operating Procedures (SOP)
- Clinical trials & study protocols
- Regulatory documentation (including Embassy legalized translations)
- Audit documentation
- CTD and Non-CTD Dossiers
- Summary of Product Characteristics (SmPC)
- PBRER/PSUR/Pharmaco-vigilance reports
- Scientific journals
- Product profiles & data sheets
- Labels, inserts, instructions & manuals
- Clinical trial reports
- Government applications / Tenders
- Marketing, promotional, and consumer education materials
We assist our corporate clients in these industries to gain valuable patient insights and achieve regulation compliance by offering a range of language services and tailor-made solutions, including:
- Text proofreading and editing
- Content analysis
- Transcription
- Interpretation
- Cost-effective
All the documents translated through LSF Medical Network are stored appropriately in our database to ensure easy tracking and editing possibilities.
The documents are Certified Translated and LSF MEDICAL NETWORK undertakes completely responsibility of the accuracy of translated content.
Our customers are additionally protected by measures in our terms of service agreement.The organization is managed in a way that assures you of the strictest confidentiality for all of your business knowledge. Our processes comply with the strict quality control standards that are necessary for pharmaceutical development.
Our pharmaceutical expertise is further supported by our additional strengths in medical device and medical systems translation.